Tramadol is a not a benign, NSAID analogue. It is a dual action narcotic acting on both the serotonin and norephinephrine reuptake system, and through its metabolite (M1) acts on the opioid mu receptor. Both actions contribute to the analgesic properties of Tramadol. Initially considered to be 10 times less potent than other opioids such as morphine, evidence is changing. Tramadol was first introduced in 1977 in Germany in I.V. form which has been found in studies to have low efficacy for pain. However, studies conducted by Johns Hopkins University in the 1990’s, at the time that Ortho McNeil was seeking FDA approval for its oral form called Ultram, indicated that high dose Tramadol acted very similarly to other opioids such as oxycodone. Like these other opioids, it is associated with dependence. Upon abrupt cessation, Tramadol users experience mild withdrawal symptoms. Ignoring this evidence, the FDA did not schedule Tramadol as in 1994, considering that it possessed the same risk as Viagra or Lipitor. After growing evidence of its contribution to opioid overdose morbidity and mortality, in August 2016, the FDA did reschedule it as a schedule IV drug.
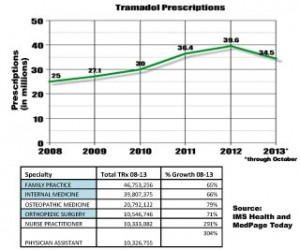
Tramadol use has skyrocketed. In 2008, 25 million prescriptions were filled. Four years later (2012), over 40 million prescriptions were being filled. In 2011, Tramadol was associated with 20,000 Emergency Department visits for opioid overdose, often in combination with other opioids. For instance, in Florida in 2011, there were 379 overdoses that involved Tramadol. Tramadol is used for non-medical purposes just like the other opioids. Reviewing statistics for 2011, the DEA estimates that Tramadol was being used recreationally by over 2.6 million individuals, many of whom have substance use disorder linked to the other opioids.
Tramadol’s effectiveness is limited. A meta-analysis indicated no significant effect on pain relief in low back pain and only modest effect in osteoarthritis. The World Health Organization considers this latter use as the only evidence-based use of Tramadol.
The FDA has issued a drug warning for Tramadol, advising not to use Tramadol in patients who are addiction prone or are a suicidal risk. FDA notes that Tramadol has serious addictive effects with other opioids and alcohol in respiratory depression.
Because of its inhibitory effects on serotonin reuptake, Tramadol has also been associated with serotonin syndrome.
Key take home lessons:
- Tramadol is not benign, and has been associated with abuse and overdose
- Tramadol has very few indications, the most notable is for treatment of osteoarthritis pain
- If you need to use Tramadol, avoid using with other opioid drugs.